NEW DELHI: You will soon be able to check if that pill you are popping in is safe and not spurious. The government plans to launch a ‘track and trace’ mechanism for top selling medicines to prevent the use of counterfeit and substandard medicines and ensure quality, sources told TOI.
In the first phase, 300 top selling drugs will print or affix barcodes or quick response (QR) codes on their ‘primary’ packaging labels. Primary is the first-level product packaging, such as the bottle, can, jar or tube that contains the saleable items. Widely sold antibiotics, cardiac, pain-relief pills and anti-allergics with an MRP of over Rs 100 per strip are expected to be included.
The move, though conceptualised a decade ago, was in abeyance due to lack of preparedness in domestic pharma industry. Even for exports, the track and trace mechanism has been deferred till April next year. 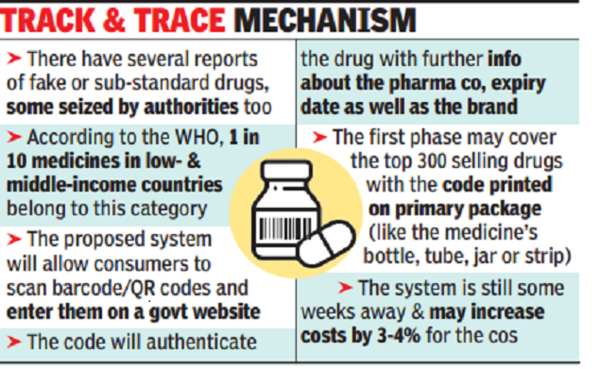
Over the years, there have been several instances of counterfeit and substandard drugs in the market, some seized by state drug regulators. Recently, among the major cases that came to light, Abbott said its thyroid medication Thyronorm that was listed by the Telangana drugs authority as “not of standard quality” was counterfeit and not manufactured or marketed by it.
In another instance, a fake drug racket of Glenmark’s blood pressure pill Telma-H at Baddi was busted. About 10% medical products in low- and middle-income countries are substandard or falsified, though these could be found in every region of the world, according to the World Health Organization.
In June, the government asked pharma companies to affix barcodes or QR codes on their primary or secondary package label that store data or information legible with software applications to facilitate authentication. Once the software is in place, consumers will be able to check the genuineness of the medicine by feeding the unique ID code on a portal (website) developed by the ministry, and later track it even through a mobile phone or a text message.
Several options are being studied, including setting up a central database agency as a single barcode provider for the entire industry, the sources said, adding it could take some weeks to implement.
“The implementation of the system will increase costs by 3-4%,” an industry player said, adding that certain companies have voluntarily started putting in the QR code.
The proposed software solution should allow manufacturers and consumers to report counterfeit pharma codes using the handheld device and the mobile application, industry experts stated.








